Medicare Program to Limit Insulin Costs to $35/month.
1/17/2025 Things to Know about Medicare Insulin Costs
Not all Medicare patients benefit from the $35 monthly cap for insulin because of specific factors related to their coverage or individual circumstances. Here are some reasons why:
- ### 1. **Plan Limitations**
- – **Medicare Part D:** Not all Part D plans immediately adopted the $35 insulin cap. Patients must enroll in a plan that participates in the Medicare Insulin Savings Program (starting in 2021) or plans aligned with cost-sharing limits under the Inflation Reduction Act (starting in 2023).
- – **Part B Coverage for Insulin:** If a patient uses insulin through a pump covered under Medicare Part B’s durable medical equipment (DME), the $35 cap should apply starting in July 2023. However, issues with billing or plan administration may cause delays.
- **Not Enrolled in a Plan That Includes Insulin Coverage**
- – Some patients may not have enrolled in a Part D plan or a Medicare Advantage plan that includes insulin benefits.
- – Patients with Original Medicare but no additional Part D coverage or who have incomplete understanding of their benefits may face higher costs.
- Issues with Plan Administration**
- – Billing errors or delays in implementing new cost-sharing policies can result in patients being charged more than $35.
- – Pharmacies may not have updated their systems to reflect the capped price.
- **Non-Covered Insulin**
- – The cap applies only to insulin included in the patient’s Medicare plan formulary. If a prescribed insulin is not covered or is off-formulary, the $35 cap may not apply.
- **Coverage Gaps or Restrictions**
- – Patients in coverage gaps or who have restrictions such as prior authorizations for certain insulin products may experience delays or increased costs until those issues are resolved.
- **Late Adoption or Implementation Errors**
- – The Inflation Reduction Act required changes effective January 2023 (for Part D) and July 2023 (for Part B), but transitional issues could delay benefits for some patients.
- What Can Be Done?
- – **Verify Coverage:** Patients should check with their Medicare plan to ensure their insulin is covered and the $35 cap is being applied.
- – **Review Formularies:** Ensuring their prescribed insulin is on the plan formulary can prevent surprises.
- – **Seek Assistance:** Medicare’s helpline (1-800-MEDICARE) or local SHIP (State Health Insurance Assistance Program) counselors can provide guidance.
- – **Pharmacy Advocacy:** Pharmacies can reprocess claims or verify that the $35 limit is being applied correctly.
- Medicare beneficiaries should contact their plan administrators to resolve discrepancies and ensure they are paying the correct amount.
Why is my medicine so expensive? What can I do about it? Jan. 7, 2020.
This is how it works; pharmacy benefit managers (PDMs) create formularies of “covered or preferred drugs”. To get on the covered formulary, drug manufacturers must give the PDM a discount (kickback). The pharmacy benefit managers then create a high  deductible and a high copay, so the patient ends up with huge out of pocket expenses. PDM’s are discouraged from listing lower priced drugs on their formularies because there is less opportunity for a discount. Follow the $.
deductible and a high copay, so the patient ends up with huge out of pocket expenses. PDM’s are discouraged from listing lower priced drugs on their formularies because there is less opportunity for a discount. Follow the $.
Everybody’s talks about the price of insulin but hardly anybody is doing anything about it.
Use manufacturer discount cards. Be sure to request these from your prescriber.
Shop pharmacy prices, they can vary quite a bit.
Request generics when available.
Consider cash price with discount card. NeedyMeds offers a drug discount card that provides a savings up to 80% on many medicines. The card is free and available to everyone. There is no registration  and your entire family can use the same card. GoodRx offers multiple discounts with its prescription discount card including prescription coupons on a prescription-by-prescription basis. Get even deeper discounts for around $5.99 per month with GoodRx Gold. Freestyle Libre retails for $142 at CVS with GoodRx Gold $112.79.
and your entire family can use the same card. GoodRx offers multiple discounts with its prescription discount card including prescription coupons on a prescription-by-prescription basis. Get even deeper discounts for around $5.99 per month with GoodRx Gold. Freestyle Libre retails for $142 at CVS with GoodRx Gold $112.79.
I searched GoodRx for cash price of GENERIC Novolog (FDA approved): 5 Flexpens of Lantus for $35. Link here. 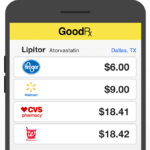
Medicare Part D
Consider cash price with discount card. GoodRx has a coupon that can be used with Medicare if paying cash. Sometimes the discounted cash price is less than the copay. The pharmacist MUST not file the prescription with Medicare in this instance.
Gabapentin for nerve pain - safe or not?

Gabapentin is often the first line of treatment for diabetic neuropathy pain. It has been around since 1993, originally approved for the treatment of epilepsy. The FDA warns that serious life-threatening and fatal respiratory problems may occur from the drug, especially if used at the same time as opioids or antidepressant/anxiety medications. Gabapentin is not an opioid and is not a controlled substance but requires a prescription. The nonopioid feature makes it a good starting point for diabetic polyneuropathy and other nerve related pain. The main treatment for diabetic neuropathy, however is control of the diabetes. Gabapentin and Lyrica work by reducing the excitement of nerves, one of the mechanisms of pain. The problem is that it works anywhere there are nerves including the brain. The most common reported side effects are dizziness, somnolence and swelling of the legs and feet. Only 20% of patients reported improvement in a study of patients taking gabapentin for diabetic neuropathy.
PROS
Generally safe when compared to other pain medications and opioids.
Generic formation available, generally cheap.
CONS
Should be used very carefully in patients with chronic obstructive lung disease COPD and elderly patients.
Should not be used with central nervous system depressions e.g. opioids, anti-anxiety medications such as benzodiazepine drugs e.g. Xanax, Valium
Tips to keep your sugar from going off the chain over the Holidays.

- Check sugar more often than usual, especially if driving or adjusting insulin or medication.
- Be party smart. At a party, try the vegetable-based appetizers 1st then carbohydrates and/or fat. Place your appetizers on a napkin instead of a plate, that limits the amount you can get at one time😊.
- Be careful with alcohol. Moderate alcohol can reduce your blood sugar, don’t drink on an empty stomach. A recommendation for alcohol intake with diabetes is “have no more than 1 drink per day for women and no more than 2 for men.” Generally, 1 drink equals 4 ounces of wine, 12 ounces of beer or 1 ounce of whiskey.
- Eat smart. You may not be able to control what food you’re served, and you’re going to see other people eating tempting treats. If you have a high carb treat, cut back on other carbs (like potatoes and bread) during the meal. If you slip up, get right back to healthy eating with your next meal.
- Be kind to yourself. Have fun. It’s okay to make mistakes, to forget things, and fall off track sometimes. It’s part of life and life isn’t perfect. Be gentle with yourself and remember that doing your best is all you can ask of yourself. The most important thing about this time of year is that you can enjoy the festivities and the company of your loved ones. Believe in yourself and show yourself the unconditional love, self-care, and nourishment you deserve! (CDC)
Do you just HATE shots? With type 2 diabetes there is something new.
 The newly available drug is called Rybelsus. It is in a class called GLP1 receptor agonist. There are several of these available on the market for injection. Examples include Victoza, Bydureon, Trulicity and Ozempic. Rybelsus Is the first one to be taken by mouth.
The newly available drug is called Rybelsus. It is in a class called GLP1 receptor agonist. There are several of these available on the market for injection. Examples include Victoza, Bydureon, Trulicity and Ozempic. Rybelsus Is the first one to be taken by mouth.
Rybelsus must be taken once daily in the morning, at least 30 minutes before food or other medications and followed by a full glass of water. It has the same warnings as the injectable GLP1 products including the potential increased risk of thyroid c-cell tumors. People who have had medullary thyroid carcinoma (MTC) or who have a family member who has had MTC are advised not to use Rybelsus or any GLP1 product. There are also has warnings about pancreatitis (inflammation of the pancreas), diabetic retinopathy (damage to the eye’s retina), hypoglycemia (low blood sugar), acute kidney injury and hypersensitivity reactions.
Rybelsus may be slightly less effective than the injection GLP1 products. It comes in 3, 7 and 14 milligram sizes. It is recommended that a patient take the lower dose for a full month before advancing to in the higher doses.
Should I take Supplements to help my blood sugar?
One study shows that cinnamon reduces fasting blood glucose, but  only by small amounts. Cinnamon in these studies reduced blood sugar by approximately 8.6 MG/DL with a range +/- 3 MG/DL. Therefore, taking cinnamon might reduce your morning fasting blood sugar by 11.6 or 5.6 MG/ DL. If morning sugar 220, at best to 208 or at worse to 214. Not much bang for the Buck$.
only by small amounts. Cinnamon in these studies reduced blood sugar by approximately 8.6 MG/DL with a range +/- 3 MG/DL. Therefore, taking cinnamon might reduce your morning fasting blood sugar by 11.6 or 5.6 MG/ DL. If morning sugar 220, at best to 208 or at worse to 214. Not much bang for the Buck$.
Herbs
Bitter Melon
- Contains compounds that mimic insulin and help lower blood sugar.
Gymnema Sylvestre
- Known as the “sugar destroyer” for reducing sugar absorption and cravings.
Aloe Vera
- Aloe vera juice or supplements may reduce fasting blood glucose.
Turmeric (Curcumin)
- Has anti-inflammatory and blood sugar-lowering effects.
Holy Basil (Tulsi)
- Helps lower blood sugar by reducing stress-induced spikes.v

Prescription drug prices diving you mad??
This information was researched from web sites of various plans. It is subject to change and I cannot guarantee accuracy.
Commercial Prescription Plan Deductibles
The most popular prescription plans in South Carolina are Blue Cross Blue Shield; South Carolina PEBA, Cigna and Express Scripts.
Deductibles for Blue Cross Blue Shield PEBA $490 individual and $980 family +$14 for physician office or video visit; $175 for emergency care; $105 for outpatient facility services.
Cigna. Deductibles for in-network and out-of-network providers: $2,000/individual or $4,000/family for out-of-network providers: $2,000/individual or $4,000/family Amount your employer contributes to your account: Up to $400/individual or $800/family.
How can I get help with out of pocket expenses with commercial insurance?
Tips for getting help with out of pocket expenses with commercial insurance.
- Ask your medical professional for discount cards.
- Shop around. Pharmacies have different prices Check with GoodRX,com for best prices in your area.
- Check with agencies that can help you with pharmaceutical company discounts. Download list here.
Medicare Part D Deductibles
Deductibles vary between Medicare drug plans. No Medicare drug plan may have a deductible more than $415 in 2019. Some Medicare drug plans don’t have a deductible.
How soon will the 2019 doughnut hole appear? Soon enough!
Initial Coverage Limit (ICL): $3,820
Out-of-Pocket Threshold (or TrOOP): $5,100
You’re in the doughnut after your total cost of medication (to the plan) reaches $3820.
You’re out of the donut hole once you have spent $5100.
TIP: Ask for cash price. Sometimes cash price is lower than Co-pay. If paying cash insist the pharmacy does NOT run it through your insurance. Medicare Part D is a voluntary program, my understanding is you can opt out for specific prescriptions at any time. If you pay cash, the retail cost of the drug doesn’t count toward the doughnut hole.
Check with Medicare.gov
How can I get help with out of pocket expenses with Medicare Part D? Download list here.
Diet soda linked to stroke.
An article published in the Journal Stroke indicated an increased risk of stroke and heart disease in women having more than 2 diet drinks per day. Women using the diet drinks were twice as likely to have a stroke or heart problem. On the day the article was released Coca-Cola stock dropped 8.38% and Pepsi 1.34%. This may not be significant when compared to stock price swings. It will be interesting to see what happens over the next several days.
The risk of stroke in diabetics (type I or 2) is 1.5 times greater than a similar person without diabetes. It is not known if that is in addition to the risk from diet drinks. People with diabetes tend to use artificially sweetened products. According to the American Diabetes Association the risk of stroke is even greater if you:
- Are older than 55 years.
- Have a family background is African-American.
- Have already had a stroke or TIA.
- Have a family history of stroke.
- Have heart disease.
- Have high blood pressure.
- You are overweight.
- Have high cholesterol.
- Are not physically active.
What’s in diet soda?
A 12 ounce can of diet Pepsi lists the following ingredients: carbonated water, Caramel coloring, phosphoric acid, potassium benzoate, sucralose, acesulfame potassium, caffeine, natural flavor and citric acid.
Note there are 2 sweeteners in this soda. Sucralose is 200 times as sweet as sugar and acesulfame potassium is 650 times sweeter. Some believe that use of highly sweet products dulls the senses to sweetening therefore making people crave sugary, high-calorie foods.
What to do? All things in moderation. A teaspoon of sugar contains about 5 g carbohydrate, a slice of bread contains about 15 g, 12 ounces of regular Pepsi 41 g. If you use 2 teaspoons of sugar in your coffee the effect on your blood sugar will be less than eating a piece of bread.
Glaucoma
Glaucoma. If you are older and or African American or have diabetes you are more likely to have glaucoma. The most common form, open-angle glaucoma, accounts for 19% of all blindness among African Americans compared to 6% in Caucasians. Glaucoma is often treated with eye drops. When you run out of your glaucoma medicine, you run the risk of increased eye pressure and damage your vision. If you’re in a bind and no drops come from the bottle, there are a few tricks for a short-term work around.
- Store your “empty” eye drop bottle upside down (we use a wine glass).
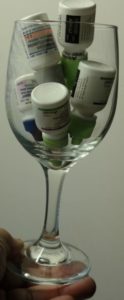
- Place the bottles in the refrigerator.
Often, when you run out of regular drops, these upside-down stored bottles will yield one or two drops. Why the refrigerator? Chemicals breakdown at a lower rate when cold therefore remain effective longer.
Diabetes Pills Action and Side Effects
Medicine | Action | Side effects |
Metformin | Lowers glucose (sugar) released from the liver | Stomach problems, usually diarrhea. |
Sulfonylureas. Glimepiride (Amaryl), glyburide (DiaBeta), glipizide (Glucotrol) | Stimulate the pancreas to release more insulin. | Low blood sugar. Long acting, if sugar is low and corrected it’s likely to go low again in several minutes. Promotes weight gain. |
Meglitinides. Repaglinide (Prandin), nateglinide (Starlix) | Stimulate the pancreas to release more insulin. | Short acting, taken just before meals, less likely to cause low blood sugar. Promotes weight gain. |
Thiazolidinediones TZDs. Pioglitazone (Actos). | Makes insulin work better. Increases carbohydrate uptake by muscle and fat. | Weight gain, swelling, fluid retention. Might make heart failure worse. |
DPP-4s. Sitagliptin (Januvia); saxagliptin (Onglyza) linigliptin (Tradjenta) | Increases insulin level but only if blood sugar is elevated. | Rare. Stomach discomfort, diarrhea |
Alpha-glucosidase Inhibitors. Acarbose (Precose); | Absorbs carbohydrate from the GI tract. | Gas, gas, gas. Rarely diarrhea, upset stomach, abdominal pain. |
Bile acid sequestrants. Colesevelam (WelChol). Pills or powder. | Controversial. It may improve insulin action by decreasing free fatty acids from the liver. Lowers LDL cholesterol | Gas, diarrhea, constipation, rarely nausea. Interferes with other medications, must be taken several hours away from other pills. |
SGLT2 inhibitors. Canagliflozin (Invokana); dapagliflozin (Farxiga); empagliflozin (Jardiance); ertugliflozin (Steglatro) | Blocks reabsorption of sugar in the kidney. This effectively pulls sugar from the blood and places it in urine. | Increased urination, genital yeast infection, urinary tract infections, ketoacidosis, dehydration. Rarely decreased kidney function; Necrotizing fasciitis* |
Bromocriptine, (Cyloset) Rarely used. | Increases brain dopamine D2 and reduces sympathetic nerve action | Nausea, asthenia, constipation, dizziness, weakness. |
*Recently the FDA issued a warning about necrotizing fasciitis of the perineum occurring in patients taking SGLT2 inhibitors. Necrotizing fasciitis, also called Fournier’s gangrene is an extremely rare but life-threatening bacterial infection of the tissues around the genitals. It generally occurs in males between ages 50 and 79 and is estimated to occur in a little over 3 patients per 100,000 taking this class of medication.
New natural product for weight loss. Plenity
lI was excited about FDA approval of a new drug for weight loss called PLENITY®. This 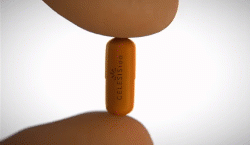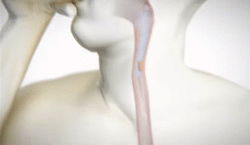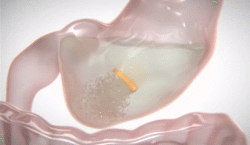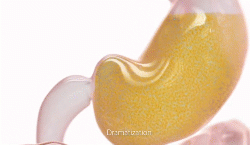 product, taken in capsules, expands with water and fills the stomach (about 25%). It is not a drug but a combination of cellulose and citric acid, natural products. A recent study published in the Journal “Obesity” showed 6.4% weight loss with Plenity over 4.4% for placebo. For someone weighing 210 pounds, that means over 13 pounds weight loss with Plenity Vs 9 pounds for placebo. Both groups were given a dietary and exercise programs for weight loss. The lesson: diet and exercise alone can produce 69% as much weight loss as this prescription product added to diet and exercise
product, taken in capsules, expands with water and fills the stomach (about 25%). It is not a drug but a combination of cellulose and citric acid, natural products. A recent study published in the Journal “Obesity” showed 6.4% weight loss with Plenity over 4.4% for placebo. For someone weighing 210 pounds, that means over 13 pounds weight loss with Plenity Vs 9 pounds for placebo. Both groups were given a dietary and exercise programs for weight loss. The lesson: diet and exercise alone can produce 69% as much weight loss as this prescription product added to diet and exercise 
The problem with all “artificial” weight loss programs is the effect doesn’t last unless there is also a persistent lifestyle change. We have many patients who had bariatric surgery or took medications like Belviq or phentermine. Most are right back where they started as the weight returned. The problem of obesity is simply consumption of more fuel that is being burned.
Drilling down into the details of Plenity®, enthusiasm decreased.
PROS
- Derived from natural products (cellulose and citric acid)
- No drugs absorbed into the body.
- Effective.
CONS
- User required to take 3 capsules before lunch and dinner with 16 ounces of water before the meal.
- Side effects involve abdominal distention, pain, constipation, diarrhea, flatulence (passing gas), infrequent bowel movements and nausea.
- Requires prescription
- Projected $$$ not published.
- No news on insurance coverage
You can do this without a prescription, but it is recommended that you get approval of your healthcare provider before starting this.
Just like Plenity, fiber expands in the stomach when water is added, the result is stomach filling. The most effective fiber forms are psyllium such as Metamucil®, Fiberall®. etc. you can even purchase generic, organic psyllium husk powder (Amazon, GNC). If one mixes 2 tablespoons of psyllium powder in 8 ounces of water, drink that and follow it with another 8 ounces of water, 20 to 30 minutes before meals, a similar effect as described above occurs. Several years ago, while practicing in Wisconsin, we performed an unofficial study with several patients utilizing this method. Of those who followed the program, 100% lost at least 5 pounds in 2 weeks. We attempted to expand the study but the company marketing the product (Fiberall®), Johnson’s Wax, refused to fund the project. The major drawback: drinking 16 ounces of water before a meal.
Disaster preparedness
Hoping that a disaster will not happen gets us into a lot of trouble. My dad used to say, “there is an inverse relationship between the likelihood of something happening and the level of preparedness for  it”. In other words, the more prepared you are, the less likely the bad event will happen. The person with no spare tire is most likely to have a flat.
it”. In other words, the more prepared you are, the less likely the bad event will happen. The person with no spare tire is most likely to have a flat.
It is a good idea to create a kit for disasters. For those of us with diabetes, small items like extra batteries, supplies to check blood sugar and items to treat low blood sugar are easily forgotten. Insulin should be in a cooler with reusable cold packs but not close to dry ice (might freeze the insulin). The Diabetes Disaster Response Coalition has created a disaster check list.
Diabetes Emergency Kit


